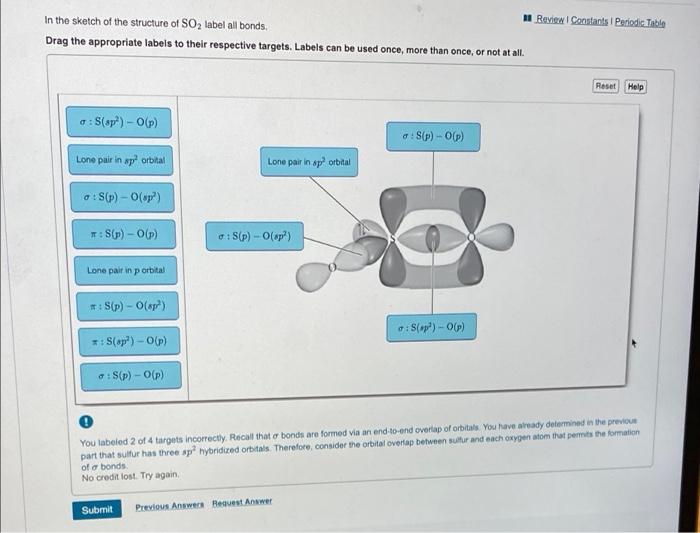
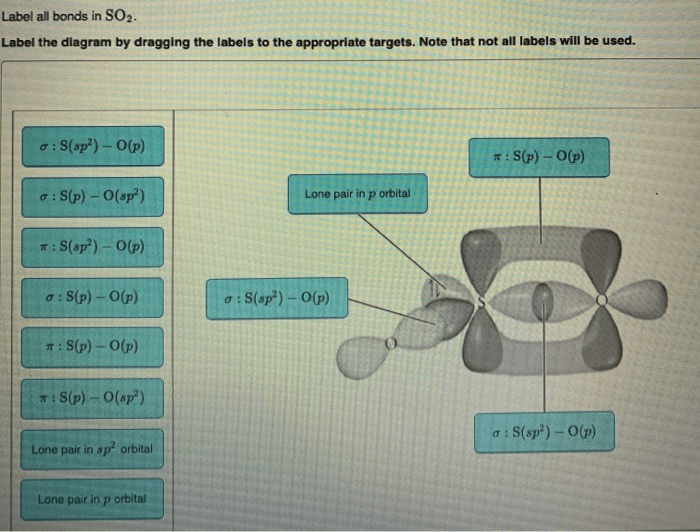
Label all bonds in so2. – In the realm of chemistry, sulfur dioxide (SO2) stands out as a molecule with a fascinating bond structure. Delving into the intricacies of its bonding characteristics, we embark on a journey to label all bonds in SO2, unraveling the molecular blueprint that governs its properties and behavior.
SO2, a polar molecule with a bent geometry, possesses two covalent double bonds between the central sulfur atom and two oxygen atoms. These double bonds arise from the sharing of electrons between the sulfur and oxygen atoms, creating a stable and symmetrical molecular structure.
Sulfur Dioxide Bond Structure
Sulfur dioxide (SO2) is a colorless, pungent gas with a molecular weight of 64.07 g/mol. It is a major air pollutant and a greenhouse gas. SO2 is formed when sulfur-containing fuels are burned.
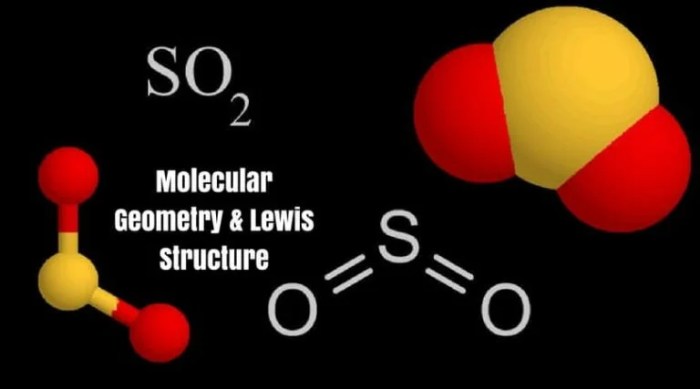
The SO2 molecule has a bent shape. The sulfur atom is bonded to two oxygen atoms by double bonds. The bond angle between the two oxygen atoms is 119.5 degrees.
Types of Bonds in SO2
The SO2 molecule has two types of bonds:
- Two double bonds between the sulfur atom and each of the oxygen atoms
- One lone pair of electrons on the sulfur atom
SO2 Bond Structure Illustration
The following diagram shows the SO2 bond structure:

In the diagram, the sulfur atom is represented by the yellow sphere, and the oxygen atoms are represented by the red spheres. The double bonds are represented by the black lines.
Bond Length and Bond Angle
The bond length between the sulfur and oxygen atoms in SO2 is 143.1 pm. This bond length is shorter than the typical S-O single bond length of 164 pm, indicating that the S-O bonds in SO2 have some double bond character.The
bond angle between the oxygen atoms in SO2 is 119.5 degrees. This bond angle is greater than the tetrahedral bond angle of 109.5 degrees, indicating that the SO2 molecule is bent. The bent shape of the SO2 molecule is due to the repulsion between the lone pairs of electrons on the sulfur atom.The
bond length and bond angle in SO2 affect the molecular geometry of the molecule. The short S-O bond lengths and the bent shape of the molecule indicate that the SO2 molecule is polar. The polarity of the SO2 molecule makes it a good solvent for polar molecules.
Bond Properties
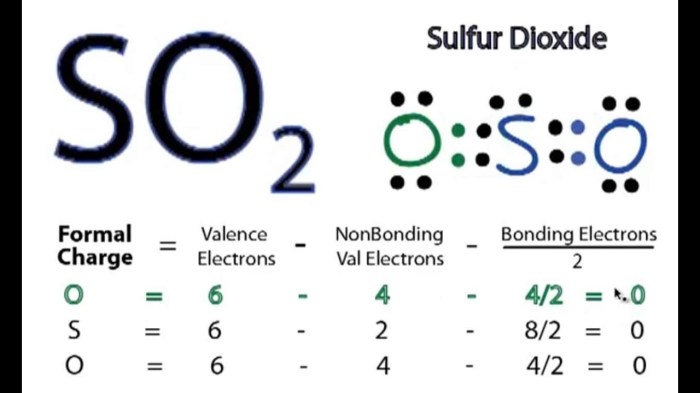
Sulfur dioxide has two S-O bonds. The polarity of these bonds is due to the difference in electronegativity between sulfur and oxygen. Oxygen is more electronegative than sulfur, so it attracts the electrons in the bond more strongly. This results in a partial negative charge on the oxygen atoms and a partial positive charge on the sulfur atom.The
sulfur atom in sulfur dioxide is sp2 hybridized. This means that it has three hybrid orbitals that are used to form bonds with the oxygen atoms. The remaining p orbital on the sulfur atom is perpendicular to the plane of the three hybrid orbitals.
Resonance in SO2
The sulfur dioxide molecule can resonate between two equivalent Lewis structures. In one Lewis structure, the sulfur atom has a double bond with one oxygen atom and a single bond with the other oxygen atom. In the other Lewis structure, the sulfur atom has a single bond with both oxygen atoms.The
resonance in sulfur dioxide results in the S-O bonds having a bond order of 1.5. This means that the S-O bonds are stronger than a single bond but weaker than a double bond.
Comparison with Other Molecules
Sulfur dioxide (SO2) shares similarities with other molecules like carbon dioxide (CO2) and nitrogen dioxide (NO2) in terms of their molecular structure and bonding characteristics. However, there are also key differences in their bond lengths, bond angles, and overall chemical reactivity.
Bond Lengths
The bond lengths in SO2 are generally shorter than those in CO2 and NO2. This is because the sulfur atom in SO2 is smaller than the carbon and nitrogen atoms in CO2 and NO2, respectively. The shorter bond lengths in SO2 indicate stronger bonds between the sulfur atom and the oxygen atoms.
Let’s get down to business and label all the bonds in SO2. This simple task can be likened to a graceful dance , where the electrons move harmoniously between the atoms. By understanding the bond types and their characteristics, we gain valuable insights into the molecular structure and reactivity of SO2.
Bond Angles
The bond angles in SO2 are also different from those in CO2 and NO2. SO2 has a bent molecular geometry, with a bond angle of approximately 119 degrees. In contrast, CO2 and NO2 have linear molecular geometries, with bond angles of 180 degrees.
Chemical Reactivity
The differences in bond lengths and bond angles between SO2, CO2, and NO2 have a significant impact on their chemical reactivity. SO2 is more reactive than CO2 and NO2 because its shorter bond lengths and bent molecular geometry make it more susceptible to attack by nucleophiles.
This increased reactivity makes SO2 a more effective reducing agent and a more potent air pollutant.
Spectroscopic Analysis
Spectroscopic techniques offer valuable insights into the bond structure and molecular properties of sulfur dioxide (SO2). These techniques probe the vibrational and rotational motions of molecules, providing information about their bond strengths, bond lengths, and molecular geometry.
Infrared (IR) Spectroscopy
IR spectroscopy measures the absorption of infrared radiation by molecules. When IR radiation is incident on a molecule, it can be absorbed if the frequency of the radiation matches the vibrational frequency of the molecule. The absorption of IR radiation causes a change in the vibrational state of the molecule, resulting in the excitation of a vibrational mode.
In the case of SO2, the IR spectrum exhibits two characteristic absorption bands corresponding to the symmetric and asymmetric stretching vibrations of the S-O bonds. These bands provide information about the bond strength and bond length of the S-O bonds.
Raman Spectroscopy
Raman spectroscopy is another technique that can be used to study the vibrational motions of molecules. Unlike IR spectroscopy, which measures the absorption of radiation, Raman spectroscopy measures the inelastic scattering of light by molecules.
When light is scattered by a molecule, a small portion of the scattered light undergoes inelastic scattering, meaning that the energy of the scattered light is different from the energy of the incident light. The difference in energy corresponds to the vibrational energy of the molecule.
The Raman spectrum of SO2 exhibits two characteristic bands corresponding to the symmetric and asymmetric stretching vibrations of the S-O bonds. These bands provide complementary information to the IR spectrum, allowing for a more comprehensive understanding of the vibrational properties of SO2.
Characteristic Vibrational Frequencies
The characteristic vibrational frequencies of SO2 are as follows:
- Symmetric stretching vibration: 1151 cm -1
- Asymmetric stretching vibration: 1361 cm -1
These vibrational frequencies are sensitive to changes in the bond strength and bond length of the S-O bonds, making them useful for studying the effects of substituents or environmental factors on the molecular structure of SO2.
Applications
Sulfur dioxide (SO2) finds various applications in industrial processes and atmospheric chemistry, while also posing environmental and health concerns.
Industrial Applications
- SO2 is used in the production of sulfuric acid, which is a key component in fertilizers, batteries, and other industrial chemicals.
- It serves as a bleaching agent in the paper and textile industries.
- In food preservation, SO2 is employed as an antioxidant and antimicrobial agent.
- SO2 is utilized as a reducing agent in various chemical reactions.
Atmospheric Chemistry
SO2 plays a significant role in atmospheric chemistry, primarily as a precursor to sulfuric acid aerosols.
- These aerosols contribute to the formation of acid rain, which can harm ecosystems and infrastructure.
- SO2 emissions from power plants and industrial activities can lead to smog and respiratory problems.
Environmental and Health Implications, Label all bonds in so2.
SO2 emissions have significant environmental and health impacts:
- Exposure to high levels of SO2 can cause respiratory irritation, asthma, and other health issues.
- Acid rain, formed from SO2 emissions, damages forests, lakes, and buildings.
- SO2 contributes to climate change by indirectly affecting the Earth’s radiation balance.
Question Bank: Label All Bonds In So2.
What is the hybridization of the sulfur atom in SO2?
The sulfur atom in SO2 is sp2 hybridized.
How does the bond angle in SO2 affect its molecular geometry?
The bond angle of 119.5° in SO2 results in a bent molecular geometry.
What spectroscopic techniques can be used to study the bond structure of SO2?
Infrared (IR) and Raman spectroscopy can provide insights into the bond structure of SO2.